How Does Soap Work?
Share
I usually find myself answering this question from two perspectives. Either, "Is soap really necessary?" or "Is soap enough?" So let's have a quick look at the chemistry of how soap really works.
At its core, what soap really does is to make water a better cleanser. By itself, water does an ok job at removing dirt. Think about trying to get dirt off your hands with a dry paper towel or by rinsing with water. Water does a much better job!
But now think about washing your hands with either a dry soap bar or soap combined with water, the dry soap bar wouldn't work at all. This is because soap and water need to work together to get things clean.
What is soap?
True soap (i.e. not detergent or disinfectant) is chemically known as a "salt". This is because soap is make by combining a strong base (sodium hydroxide or potassium hydroxide) with a weak acid (fatty acids in e.g. lard and tallow) which undergo a chemical reaction to make a new sodium or potassium compound. [1] If you want more details on that process, check out my article What is lye and why is it in our soaps? [2]
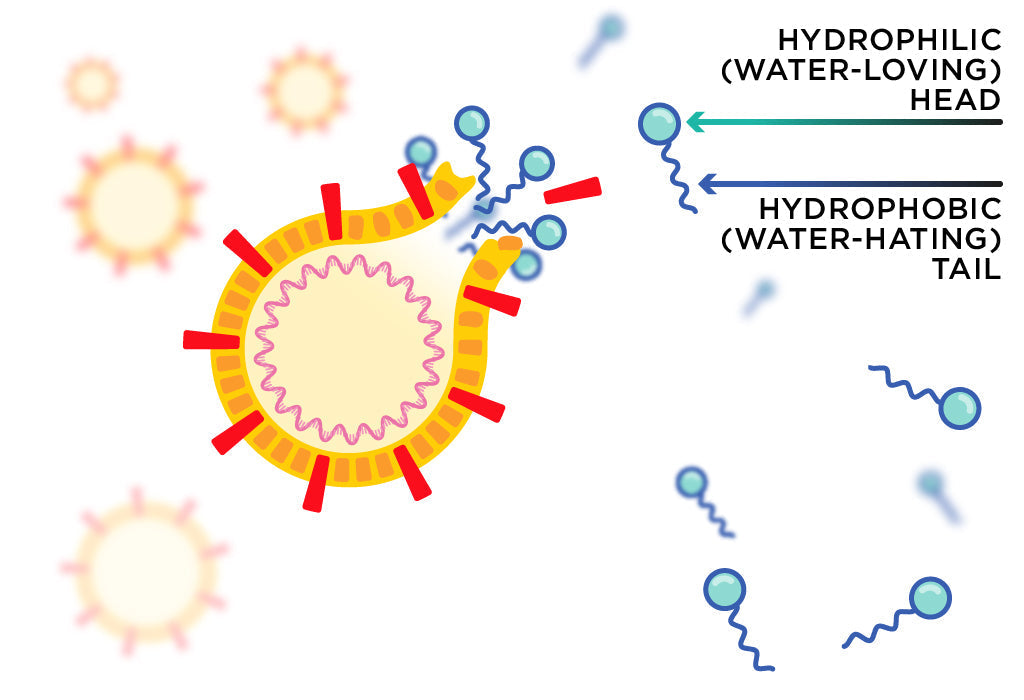
What does soap do?
Remember high school chemistry? Yeah, I didn't either! When I started making soap I had to learn the basics of chemistry all over again. Where soap is concerned, you need to understand that the molecules involved all have things they're attracted to and things they're repelled from. Water molecules, for example, are attracted to each other in a way that excludes fat and oil molecules. This is why oil and water don't mix.
In the case of hand washing, we need to know that many disease causing organisms protect themselves with a lipid barrier. Lipid is just a nice catch all word that includes fats and oils! So if you have germs on your hands and just rinse them with water, that lipid barrier will prevent them from being washed away.
Enter soap! When soap is made, the molecules formed are little chains with a charged sodium ion on the end. This means that one end of the chain ends up attracted to water and the other end is repelled by water. The end that is repelled by water is attracted to lipids, so you end up with clusters of soap molecules that attach themselves to oils and germs on one end and to water with the other, allowing you to rinse the whole mess off of your hands and down the drain! [3]
Is soap necessary?
If you're someone with sensitive skin or simply want to avoid unnecessary chemicals, you may find yourself wondering if you even need soap. Repeated washing, even with the mildest soap, can definitely dry out your skin but never using soap leaves you open to every cold, flu, corona virus or stomach bug that is going around.
I'm not someone who feels like I need a full head to toe soap scrub every day and I think my skin is generally happier for that. Your skin contains lipids too and soap doesn't differentiate between the lipids in your skin and the lipids in grease and germs. As with most gentle soap, my soaps are formulated to have enough extra fat (in my case lard and/or tallow) that some is left behind on the skin.
But your skin is also host to many beneficial organisms which can be harmed by constant washing. The balance I strike is that most of my skin stays happiest with only occasional soaping but hand washing is one of the best barriers against getting sick!
Is soap enough?
On the other end of the spectrum are folks who are very aware of infectious microbes and worry that soap doesn't effectively kill them. Many companies play on these fears with various sanitizing and antimicrobial products.
The worst one is antibacterial soap. Did you know that regular soap and water are just as effective as antibacterial soap at killing germs? In fact the US Food and Drug Administration has banned antibacterial chemicals like triclosan from non-prescription soaps, saying that there "isn't evidence to show that over-the-counter (OTC) antibacterial soaps are better at preventing illness than washing with plain soap and water." [4]
Hand sanitizer is a very effective product that lets you clean your hands on the go when you may not have access to water for hand washing. Like soap, the alcohols in hand sanitizer interfere with the lipid barrier on microbes. Rather than clustering around them so they can be washed away, alcohols kill the organism.
This doesn't make hand sanitizer more effective than soap and water though. If you've already washed your hands, sanitizer is redundant and not necessary, not to mention that it is much more drying for your skin!
So yes, plain soap and water are very effective at cleaning your hands, including removing and destroying infectious organisms. The real key, whether for hand washing or using sanitizer, is good technique. Germs can hide in all kinds of nooks and crannies, so full coverage around nails and in between fingers is key!
History of Hand Washing
The idea of hand washing for cleanliness is surprisingly new, dating only to the 1840s. Before this time, soap was used for cleaning dishes and textiles, for removing visible dirt from the hands or in many cultures as part of a religious or spiritual ritual to cleanse the hands for sacred rites.
Read more about the history of hand washing here!
Resources:
- https://en.wikipedia.org/wiki/Soap
- https://fatchancesoap.com/blogs/news/what-is-lye-and-why-its-in-our-soaps
- https://www.science.org.au/curious/people-medicine/hand-sanitiser-or-soap-making-informed-choice-covid-19
- https://www.fda.gov/consumers/consumer-updates/skip-antibacterial-soap-use-plain-soap-and-water

